Abstract
Background: ABO non-identical transfusions have been associated with increased risk of hemolytic transfusion reactions. We previously reported that the ABO immune complexes (ABO-IC) formed in vitro as a result of ABO non-identical exposure affect platelet function, red blood cell integrity, and clot kinetics (Vox Sang. 2016;110:219-26). We developed an in vitro model as a means to investigate the interaction between ABO-IC and endothelial cells (EC).
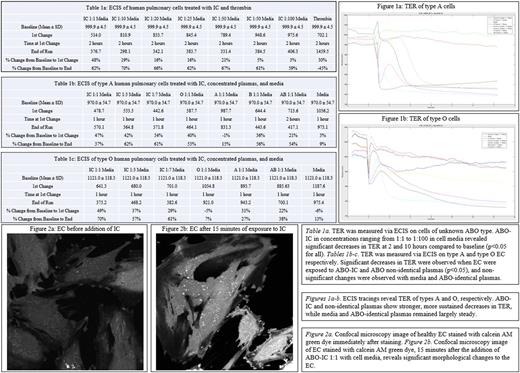
Methods: The ABO-IC were prepared by combining equal volumes of concentrated O plasma (high titers of anti-A and anti-B) with A plasma (high concentration of A antigens). Anti-A titers were measured before and after mixing to confirm the presence of ABO-IC. ABO-known human pulmonary vessel EC were then seeded onto gold microarray plates to prepare for electric cell-substrate impedance sensing (ECIS). The trans-endothelial electrical resistance (TER) was recorded for 48 hours after the addition of media, PBS, thrombin, O, A, B, and/or AB plasmas added 1:1 with media, as well as various concentrations of ABO-IC. Statistics were performed using the chi-square test to compare TER values at various timepoints to baseline. Human pulmonary EC were cultured on glass-bottomed petri dishes for confocal microscopy. The cells were stained with fluorescent green calcein AM dye and assessed for viability at 100x magnification at baseline and again 15 minutes following the addition of ABO-IC.
Results: Substantial decreases in TER were observed over the course of 2 hours in O and A EC when treated with non-identical plasma, and sustained over 10 hours (p<0.05 for all). Significant decreases in TER were also observed in cells treated with various concentrations of ABO-IC, over 2 hours and were found to be dose-dependent and independent of ABO type (p<0.05 for all). Unlike the ABO-IC, exposure to thrombin revealed a marked decrease in TER after 2 hours, although the final TER measurement revealed complete recovery (Table 1a-c). Incubation with ABO-identical plasma showed slight decreases in TER over time that were not statistically significant when compared to incubation with cell media (Figure 1a-b). Physical damage to the EC membrane were observed via confocal microscopy after 15 minutes of exposure to ABO-IC (Figures 2a-b).
Discussion: The exposure of ABO-IC to human pulmonary vessel EC revealed significantly decreased monolayer integrity via ECIS and confocal microscopy. The decreases seen in the TER of EC incubated with non-identical plasma and ABO-IC indicate the potential for capillary leak as a possible reaction to ABO non-identical transfusion. We have shown that the substantial decrease in TER when exposed to plasma is ABO type-dependent, and that the damage caused to the cells happens within a few hours and is irreversible over 48 hours. The confocal images reveal a significant change in the morphology of the EC membrane within 15 minutes of exposure to ABO-IC. These preliminary data suggest that anti-A and ABO-IC may significantly affect EC viability and function. This in vitro model provides potential novel mechanisms for the detrimental effects of ABO non-identical transfusion on EC. Further investigation of biomarkers for cell viability and integrity in response to exposure to ABO-IC is underway.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal